Partnering for Progress: Enabling Radiopharmaceutical Innovation Through CRO Collaboration
Author: Laura Nicols
Radiopharmaceutical therapy (RPT) represents a transformative approach to cancer treatment, harnessing the power of radioactive isotopes to selectively target and destroy malignant cells. Unlike conventional external beam radiation therapy—which non-selectively irradiates both healthy and cancerous tissues—radiopharmaceuticals consist of a radioactive isotope conjugated to a biologically active carrier molecule that binds specifically to receptors or antigens expressed on tumour cells. This targeted mechanism enables a more precise therapeutic effect while minimizing systemic toxicity.
Positioned at the intersection of medicine, chemistry, and nuclear physics, radiopharmaceuticals are increasingly integral to both diagnostic imaging and therapeutic strategies. In nuclear medicine, diagnostic radiopharmaceuticals such as those used in PET (positron emission tomography) and SPECT (single-photon emission computed tomography) facilitate early disease detection and real-time evaluation of biological processes. These diagnostic capabilities lay the foundation for radiotheranostics—a growing research space where the same molecular targets used for imaging are leveraged for therapy.
Radiotheranostics enables a personalized, image-guided treatment approach. Radiotracers labelled with therapeutic isotopes (e.g., lutetium-177, actinium-225) can be tailored to deliver cytotoxic radiation directly to cancer cells identified via diagnostic scans. This dual-use strategy not only enhances treatment specificity and reduces off-target effects, but also allows for adaptive therapy, in which treatment regimens are modified in real-time based on imaging feedback and biological response.

Current Landscape and Market Growth
As of 2024, the global radiopharmaceuticals market is estimated at USD 10.46 billion. It is projected to reach approximately USD 17.39 billion by 2032, growing at a CAGR of 9.47% from 2025 to 2032. This rapid growth is driven by increasing investment in nuclear medicine, the expansion of theranostic applications, and a surge in demand for personalized oncology treatments.
Geographically, North America dominates the market, accounting for approximately 35.93% of the global share in 2024, with Europe holding over 23% of the global revenue, with the UK market valued at USD 353.09 million in 2024, and projected to grow at a CAGR of 8.38% these growth projections and valuations underscore both rising clinical interest and broadening commercial participation in this space.
Radiopharmaceuticals in Clinical Trials
Recent years have seen a substantial increase in the number of clinical trials investigating radiopharmaceuticals, particularly in oncology. Notable developments include:
- Growth in 225Ac-PSMA compounds in prostate cancer building on approvals in the therapeutic area of 177Lu labelled compounds. The 225Ac products look to leverage the high-energy alpha emissions of actinium-225 for targeted therapy.
- AlphaMedix™ (Lead-212-DOTAMTATE) has received FDA breakthrough designation for treating rare neuroendocrine tumors, combining lead-212 with a somatostatin analog to deliver targeted alpha radiation.
- Emerging alpha emitters, including actinium-225 and thorium-227, offer higher linear energy transfer (LET) and increased cytotoxicity for resistant tumours, and are being studied in early-phase trials for glioblastoma, hematologic malignancies, and advanced solid tumour such as 225Ac-DOTA-SP which targets the NK-1 receptor using substance P labeled with actinium-225, aiming to treat aggressive brain tumors like glioblastoma through targeted alpha therapy.

Additionally, radiopharmaceuticals are being integrated into basket and platform trial designs, allowing for more efficient evaluation of efficacy across multiple tumour types sharing a common molecular target.

Looking Ahead
Radiopharmaceuticals, particularly in the context of radiotheranostics, are poised to reshape the landscape of precision oncology. By combining targeted imaging with therapeutic delivery, they enable more effective, individualized treatment strategies and hold promise for previously intractable cancers. Continued advancement in isotope production, regulatory frameworks, and trial design will be essential to fully realize the potential of these agents in routine clinical practice.
About Aixial Group
Since 1999, Aixial have performed more than 1,600 studies across the globe with our teams in APAC, Europe, and North America.
Our experience portfolio includes 45 radiopharmaceutical studies and we have been involved in radiopharmaceuticals development since 2010 and have successfully managed 18 Phase I, 14 Phase I/II, 3 Phase II, 4 Phase II/III, 3 Phase III and 3 Phase IV trials. Isotopes within our portfolio include, but are not limited to 89Zr, 225Ac, 11C, 14C, 64Cu, 18F, 58Fe, 131I, 177Lu, etc.

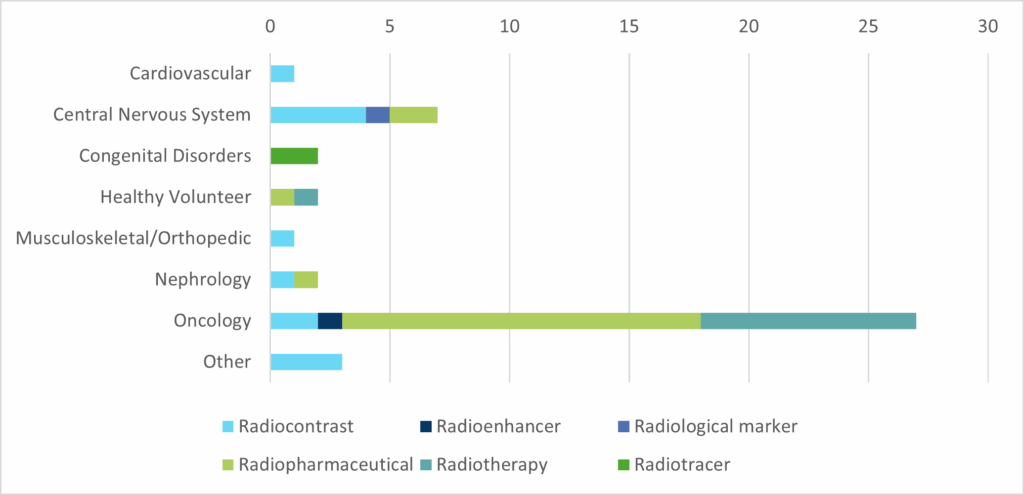
Our teams understand and appreciate that the clinical development of radiopharmaceutical agents is inherently complex, requiring seamless coordination across nuclear medicine, regulatory affairs, radiochemistry, logistics, and imaging sciences. As radiopharmaceutical research continues to expand globally, we work collaboratively with our Sponsor partners playing a pivotal role in navigating these multidisciplinary challenges and accelerating time-to-market.
At Aixial Group we offer deep, specialized expertise across the full development lifecycle of radiopharmaceuticals—from early-stage planning and regulatory strategy to clinical trial execution and seamless patient planning and recruitment. Our team understands the distinct regulatory pathways, imaging biomarker integration, radiation safety protocols, and supply chain considerations unique to these advanced therapeutics.
In addition to scientific and operational excellence, we leverage our extensive global network of qualified nuclear medicine centres and specialist research sites. These established partnerships enable streamlined patient recruitment, site activation, and protocol compliance—critical factors in radiopharmaceutical trial success.
As a leading CRO in the radiopharmaceuticals sector, Aixial Group provides the infrastructure, insight, and agility required to support sponsors in delivering complex, international clinical trials. Our track record demonstrates our commitment to advancing targeted radiotherapeutics and radiotheranostics, with a focus on quality, innovation, and speed.

How can support your next project?
Whether you’re looking for a protocol review or a proposal,
simply reach out to us by filling our request for proposal.
