Aixial Group: Experts in Radiopharmaceutical Development
Radiopharmaceutical therapy utilizes radioactive atoms to target and precisely treat cancer cells. Unlike external beam radiation which damages all cells in its path, radiopharmaceuticals consisting of radioisotopes (radioactive substances) bound to a pharmacologically active carrier molecule (tracer) specifically target and bind to or accumulate within cancer cells. Radiopharmaceuticals provide a novel way to deliver cytotoxic radiation specifically and directly to cancer cells, targeting precise signaling mechanisms, while avoiding difficulties with identification of cancer cells which can be observed with other types of therapy[1].
The worldwide market for radiopharmaceuticals was valued at over $5.2 billion in 2022. Experts predict significant growth in the coming decade, with the market reaching approximately $13.67 billion by 2032, representing a compound annual growth rate of over 10%[2]. From 2021 to 2023, Aixial’s own research indicated a 200% increase in the number of Sponsors involved in the development of radiopharmaceuticals.
Aixial experience in Radiopharmaceutical
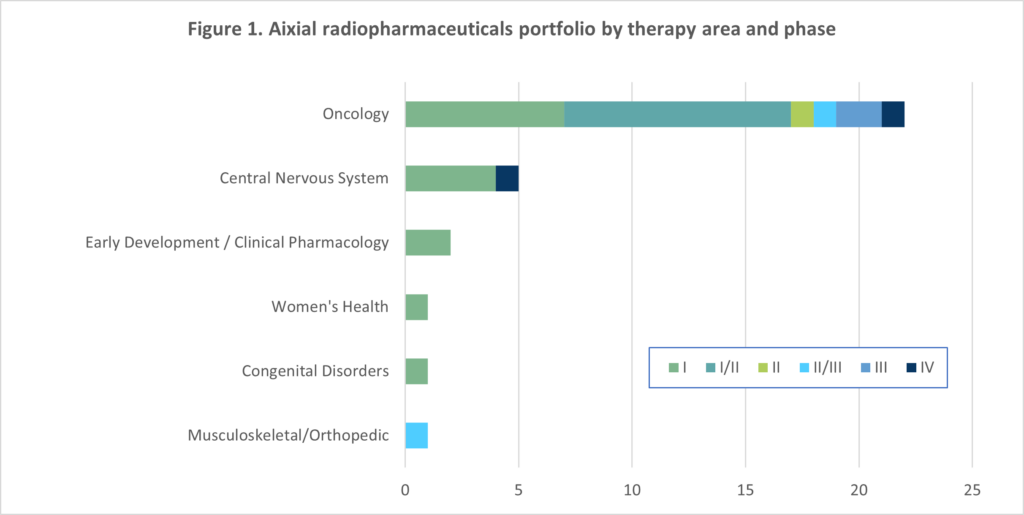
Based on customer feedback, Aixial are viewed as a CRO leader in radiopharmaceutical development. Over the last ten years, Aixial has successfully managed 32 radiopharmaceutical trials in a variety of indications, with 22 of these in oncology (see figure 1) involving numerous radioisotopes. Our portfolio of work has involved both diagnostic and therapeutic products including nanoparticles and theragnostics, hence our Subject Matter Experts (SMEs) have the required expertise to support the full spectrum and conduct of radiopharmaceuticals trials. The following chart is provided to identify our experience in radiopharmaceuticals by therapeutic area and phase.
Understanding the multidisciplinary approach in radiotherapy
Developing radiopharmaceuticals is a multidisciplinary endeavor involving nuclear medicine, radiology, pathology, oncology, dosimetry, and radionuclide imaging, amongst others. In addition to this group of practitioners, clinical trials may also involve a dynamic team comprised of a clinician, nurse, physicist, radiopharmacist, radiographer and administrative staff working together in a detailed choreography depicted below:
Disciplines involved in radiopharmaceuticals clinical trials:
Close coordination among site interdisciplinary team.

Oncology
Medical Oncologist
Clinical Coordinator

Radiation Safety Committee
Radiation Safety Officer
Health Physicist
Infusion center and patient rooms
Nuclear medicine physician
Nurses
Supporting staff
Nuclear medicine or radiology
Nuclear medicine physician
Medical physisit
Nuclear medicine technologist
Hot lab or Radiopharmacy
Nuclear medicine physician
Medical physisit
Nuclear medicine technologist
Orchestrating this choreography requiresstrong relationships and open communication between medical monitors, investigators, radiopharmacists, physicists, Nuclear Medicine Physicians, and other site departmental representatives. In view of the multi-disciplined resources involved, Aixial’s global team embrace a collaborative approach with all stakeholders. Based on our expertise in the conduct of these studies, we are happy to consult in the design of a protocol to ensure that the protocol can be operationalized and successfully conducted. Hereunder, we highlight key factors that are critical for successful execution of radiopharmaceutical protocols.
- Feasibility
- We conduct very specific and detailed site feasibility ensuring all site departments are consulted and the process flow of IMP, patients, assessments, and samples will be managed with extreme and consistent precision.
- During feasibility and site qualification, we confirm site standard practices for infusion, injection, or inhalation and check to ensure they have appropriately calibrated equipment and mandatory processes to align with IMP administration instructions.
- Sites often have preferred suppliers and/or process mandated equipment for administration of such radio products, and we will confirm if this is suitable for use in the study, or if different equipment will be required. For example, we confirm that sites have specific catheters, pumps and adaptors if they are required, and conversely, we will let sites know if ancillary infusion supplies are not needed.
- At initial stages of site qualification and initiation, the Aixial team will ensure that all site equipment is certified and validated as this can become rate limiting.
- Regulatory:
- Despite the continued rise of radiopharmaceuticals in development, there is a scarcity of documented guidance for the use of radiopharmaceuticals both in the pre-clinical stage and as products enter the clinical stage[3].
- Radiation safety issues need to be emphasized in the form of rigorous regulatory oversight to support safe and effective use, and their remains a need for harmonization of guidelines and regulations on a global scale[4].
- Based on these aforementioned factors, it becomes critical for sponsors to have expertise ‘in-house’ through their own experts, or to obtain support from a CRO with recent and relevant experience managing global radiopharmaceuticals trials. Considering the complexity of these trials, experienced team members are imperative.
- Through the performance of clinical trials on a global scale, Aixial has the required regulatory submission experience and, importantly, can offer the strategic regulatory guidance required to successfully navigate the most expeditious, fast-track, approvals processes with the US FDA and EU EMEA, including both country-level and site-level radiation committees.
- Aixial can support all required submission processes including CTA, IND, NDA, MAA and under the centralized, decentralized, mutual recognition and national European procedures.
- Site Support
- Provide the required training at the onset of the project; for example, in addition to routine SIV training, sites need explicit understanding regarding which samples are to be analyzed and reported locally.
- Define a reconciliation process for central lab samples and imaging. The communication pathway to the site management teams and sites is a critical aspect of radiopharmaceuticals trials.
- Ensure data entry expectations e.g., real-time data entry during specific study phases, are established with sites, especially critical during dose escalation phase to evaluate the safety of each cohort.
- Vendor Engagement:
- Early engagement with vendors such as imaging, dosimetry, and central labs to understand study and site requirements.
- Provide the required training at the onset of the project; for example, in addition to routine SIV training, sites need explicit understanding regarding which samples are to be analyzed and reported locally.
- The enlistment of an established imaging vendor who have extensive site training and a user-friendly portal for scan uploads is vital aspect of these unique studies. Sites often experience unexpected uploading errors, and troubleshooting these issues with them early during the set-up phase will avoid delays downstream. We are happy to provide the names of our qualified imaging vendors astute in the conduct of radiotherapeutic trials, if desired.
- Providing investigators and their staff with direct contact information and 24/7 availability.
Management of Investigational Product (IP) supply and logistics
For studies involving the use of radioactive products, an elevated level of logistical management is required throughout the product lifecycle. Aixial’s experience in radiotherapeutics has gained us valuable knowledge and agility around IP logistics that are extremely time sensitive. This key part of the process is a “race against the clock” challenge that must be met with caution, close communication, and careful planning to move the product through the supply chain seamlessly, ensuring the final product remains viable for administration to the patients.
Aixial assigns a dedicated Logistics Coordinator to each Radiopharmaceutical study we are entrusted to manage. Logistics Coordinators are senior members of our clinical team utilized to track IMP (ordering, manufacture, shipping, and receipt), patient visits and assessments, as well as sample tracking which allows trial sites to manage randomization, inventory, and IP dispensation in real-time. The Logistics Coordinator also supports sites to seamlessly integrate across the preferred Data Management Platform being utilized.
Due to the logistics of IP manufacturing and radio decay, we strongly recommend sites set-up patient scheduling up-front and early. Close planning and logistics management is needed for per-patient planning and scheduling for sample collections, imaging, and analysis. Aixial is accustomed to working with site teams at this detailed level of patient planning and coordination to ensure visits run smoothly.
While the exact logistical requirements will be study and product-specific based on the manufacturing process, manufacturing requirements, and product degradation, Aixial teams apply knowledge gained from our experience to successfully manage the handling process of radioactive materials.
Logistical oversight and management of the supply chain in this process are critical. Each step, from requesting a manufacturing slot to transportation and receipt of the product, through to administration to the patient is crucial for patient safety and proper execution of the study. The following considerations should be made in relation to any IP with a short shelf-life:
- Ensure specialized couriers are used for IP distribution to avoid unnecessary delays. Couriers tend to have defined areas of strength in specialized handling and shipping of radioactive IP, and a clear understanding of customs complexities for such products; hence we have approved vendor relationships with global couriers for bespoke supply and handling.
- Conduct dry-runs for each site to ensure shipment is within required timeframe (often incorporated into the calibration process).
- Ensure the feasibility analysis pays special attention to the selection of sites where delivery of the IMP does not create logistical difficulties and risk for on-time delivery.
Manufacturing to meeting IP Requests
Aixial suggests that enrollment be mapped out for all visits during the screening period to work within visit windows to enable all IP requests to be fulfilled. Further strategy for risk mitigation include:
- Clear capacity information provided from the outset and on an ongoing basis.
- At least 3-months’ notice of any change to manufacturing schedule (scheduled shut-downs, holiday periods, etc.).
- Sites should be made abundantly aware of risk to treatment if visits are delayed/missed.
- Sites need to be trained on and understand the manufacturer’s IP Quality Control process and the importance of reviewing and filing the Certificate of Analysis.
Aixial Group experts in Radiopharmaceutical development
Development of a novel radiopharmaceutical agent through clinical evaluation is complex and spans multiple domains; CROs provide a crucial contribution in managing the continued growth of radiopharmaceuticals development globally. Aixial possess the necessary expertise to manage the unique complexities of developing a radiopharmaceutical product from the concept stage through to trial execution and approval. In addition to the factors outlined above, Aixial leverages longstanding relationships with our global and expansive network of nuclear medicine centers and specialist research sites to facilitate patient recruitment.
As a leading CRO in the radiopharmaceuticals market, Aixial offers the knowledge and infrastructure required to deliver successful outcomes in global trials involving radiopharmaceuticals.
Contact us for a feasibility analysis offered at no cost to kick-start your plans for your next radiopharmaceuticals trial.
How can we support your next project?

[1] Sgouros, G., Bodei, L., McDevitt, M.R. and Nedrow, J. R. (2020). Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nature Reviews Drug Discovery. 19, 589-608.
[2] https://www.precedenceresearch.com/radiopharmaceuticals-market#:~:text=The%20global%20radiopharmaceuticals%20market%20size,forecast%20period%202023%20to%202032.
[3] Korde, A., Mikolajczak, R., Kolenc, P., Bouziotis, P., Westin, H., Lauritzen, M., Koole, M., Herth, M.M., Bardiès, M., Martins, A.F., Paulo, A., Lyashchenko, S.K., Todde, S., Nag, S., Lamprou, E., Abrunhosa, A., Giammarile, F. and Decristoforo, C. (2022). Practical considerations for navigating the regulatory landscape of non-clinical studies for clinical translation of radiopharmaceuticals. EJNMMI Radiopharmacy and Chemistry. 7(1):1-29.
[4] Kaushik, D., Jangra, P., Verma, R., Purohit, D., Pandey, P., Sharma, S. and Sharma, Ra. (2021). Radiopharmaceuticals: An insight into the latest advances in medical uses and regulatory perspectives. Journal of Biosciences. 46(1): 1-25.
