Regulatory Affairs
We can support on your projects all along the lifecycle of the products to ensure compliance withe the regulatory requirements and timely approval in a worldwide range.
Regulatory affairs
Regulatory affairs is the domain that monitors the way food, drugs, cosmetic products, medical devices are developed, tested, manufactured, marketed and distributed in order to certify that they meet regulatory standards for human and animal use.
Regulatory Affairs plays a crucial role in the health products industry from the early developpement phases throughout the life cycle of the health products, where they are responsible ensuring manufacturer’s compliance with applicable laws and regulations as well the safety and efficacy of health products.
We can support on your projects all along the lifecycle of the products to ensure compliance withe the regulatory requirements and timely approval in a worldwide range. Our team ensure a close partnership to provide a personalised offer in accordance with regulatory requirements and in compliance with your processes.
Our expert teams have over 25 years of regulatory knowledge and experience in global level to viable strategies and efficient development pathways thanks to 3 key advantages:

Full industry scope
We are specialised in all facets of pharmaceutical and medical device development and regulation.

FDA, EMA and International
We combine extensive scientific and regulatory experience to achieve successful outcomes with regulators across continents

Full life cycle support
From early-stage development through to approval and marketing, our regulatroy affair consultants supports our clients to meet regulatory needs across the entire life cycle of products
Solutions by Industry
We are able to support our clients on the overall regulatory activity’s initial submission and life cycle management (Renewals/ administrative, quality and safety variations), MAH extensions, MAH withdrawals) on EU, US and all International regions, in compliance to the regulatory requirements and in respect to the timelines and strategies defined. A non exhaustive list of activities is provided herafter.
Pre-Marketing Authorisation:
- Regulatory intelligence, strategies and advices within all regions
- Due diligence and compliance
- Scientific advice and agency consultation management
- Regulatory Project Management and Follow up from the beginnig to the approval of the project
- Market access
- Clinical Trial Applications
- Authoring of IND/IMPD dossier
- Authoring of complete or update of Modules 1-5 as applicable to your products
- Authoring of complete or update and submission of CEP, ASMF
- Responses to Questions of the Competents Authorities
- Create or update, review and validation of initial labelling, Art-works, CCDS, CCSI, SmPC, PIL and other related documents
- Management of Renewals
- Management of Variations (administrative, quality and safety)
- Advertising and promotional materials: Create, review, validate and submit to health authorities all advertising and promotional materials
- Annual Product Review
- Management of Change controls
- Dossier Management, publishing and submissions
- Infomed
We support our clients on the overall activities for the Registration of medical devices in Europe, US and Internatial Regions in order to place in the market medical device as fast as possible and in compliance to the regulatroy requirements. A non exaustive list of acitivites is provided herafter:
- Regulatory intelligence, strategies and advices within all regions
- Due diligence and compliance
- Classification of the Medical Devices
- Definition of the list of documents, tests, studies to be provided in compliance with the regulatory regquirements
- MDR & IVDR technical file remediation and compilation (EU 2017/745) & (EU 2017/746) and CE marking and maintance
- Quality Management system in accordance with ISO 13485
- Risk Management support
- Management of Risk Analysis / Risk assesssments
- Management of complete or update technical file
- Management and writing of PSURs / PMS / PMCF / PMPF
- Management and writing BER
- Management and writing of CER – PER / CEP – PEP
- Support on Labeling, Advertising and Promotional materials
- Support on Notified bodies Audits
- 510(k) submission
- Support on MDSAP Audits
- Internal Audits
We support our clients on the overall regulatory activity’s initial submission and life cycle management (Renewals/ administrative, quality and safety variations), MAH extensions, MAH withdrawals) on EU, US and all International regions, in compliance to the regulatory requirements and in respect to the timelines and strategies defind for the veterary medicines. A non exhaustive list of activities is provided herafter:
Pre-Marketing Authorisation:
- Regulatory intelligence, strategies and advices within all regions
- Due diligence and compliance
- Regulatory Project Management and Follow up from the beginnig to the approval of the project
- Market access
- Authoring of complete or update of Part I – IV as applicable to your products
- Authoring of complete or update and submission of CEP, ASMF where applicable
- Responses to Questions of the Competents Authorities
- Create or update, review and validation of initial labelling, Art-works, PIL and other related documents
- Management of Renewals
- Management of Variations (administrative, quality and safety)
- Advertising and promotional materials: Create, review, validate and submit to health authorities all advertising and promotional materials
- Annual Product Review
- Management of Change controls
- Dossier Management, publishing and submissions
We support our clients on the overall regulatory activity’s initial submission and life cycle management on EU, US and all International regions, in compliance to the regulatory requirements and in respect to the timelines and strategies defind for the food supplements.
- Regulatory Intelligence, strategies and advices
- Analyses of Regulatoray Requirement
- Defintion of stategies in fonction to the project
- Definition of the list of documents, tests, studies to be available in compliance with the regulatory regquirements
- Writing or update of the technical file
- Validation of the claims and Labeling, in compliance to the regulatory requirements
- Preparation of the notification/registration dossier in compliance to the local regulatory requirements
- Notification / Submission to the competent authorities
- Follow up and update of the dossier as necessary
We support our clients in a full range of Regulatory activities for the Notification and Registration of their Cosmetic Products in EU countries and International Regions.
- Regulatory intelligence, strategies and advices within all regions
- Validation and analysis of the qualitative and quantitative ingredients in the formualition
- Definition of the list of documents, tests, studies to be available in compliance with the regulatory requirements
- Definition of stategies in fonction to the project and validate with the client
- Writing PIF Part A
- Writing PIF Part B -toxicological expertise
- Validation of the claims and Labeling, in compliance to the regulatory requirements
- Preparation of the notification/registration dossier in compliance to the local regulatory requirements
- Notification CPNP
- Follow up and update of the dossier as necessary
We provide regulatory solutions and assistance throughout the life cycle of drug products, medical devices, cosmetic products and food supplements (pre-MA and post-MA activities). Our teams ensure a close partnership to offer a personalised response in accordance with regulatory requirements and in compliance with your processes.
Merita, regulatory affairs expert
Our delivery methods

Technical assistance
Technical support, advice and expertise

Platform
A range of services dedicated to your activities driven by our team

Projects
Perfomance with commitment to results
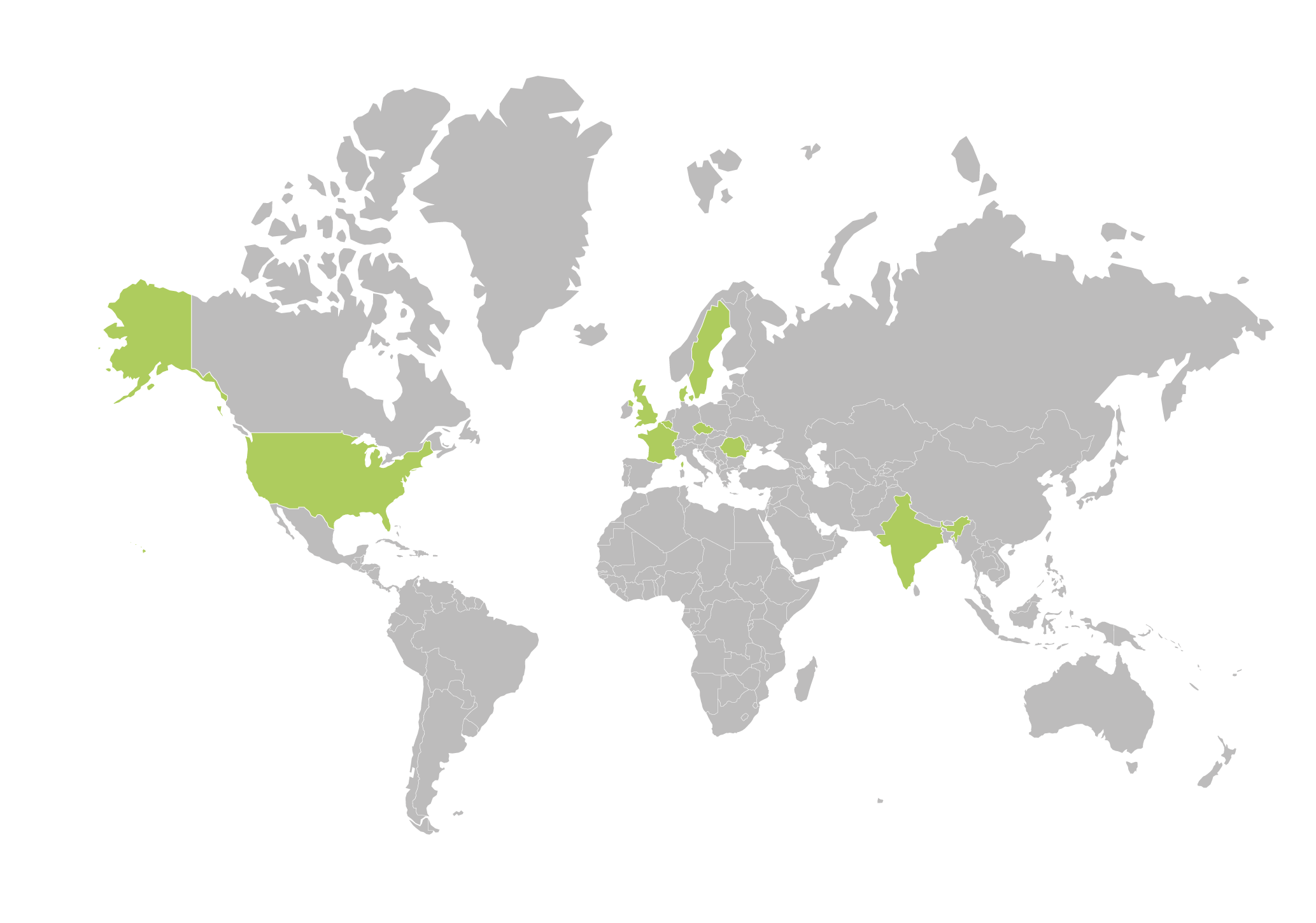
Aixial Group around the world
Aixial Group can help you around the world. We provide services onshore, nearshore, and offshore to ensure that your solution is customized to your specific needs.
Our sectors

Pharmaceutical

Food
Biotech & Vaccines

Medical devices

Veterinary

Cosmetic
How can we support your next project?

OTHER WAYS WE SUPPORT YOU
