Clinical Trial Operations
Clinical Operations play a major role in ensuring safe and ethical clinical trials, helping to spur the development of new life-saving drugs and medical devices, benefiting millions of people worldwide.
OUR PEOPLE AND PROCESSES ARE DESIGNED TO DELIVER
We look after the planning and timely management of your research, ensuring the coherence and success of entrusted projects. We prioritise transparency, managerial documentation, clear communication, dynamic risk management, and effective escalations for our clients.
Clinical Trial Operations
From the production of active pharmaceutical ingredients to the global distribution of experimental drugs to patients/subjects in a challenging pharmaceutical and clinical development environment (regulatory – clinical protocol – forecasting – manufacturing – product flows – packaging campaigns – distribution – traceability – reconciliation).
Global study manager
The Global Study Manager is responsible for the coordination of studies in relation with the several actors of the involved structures, as well as for leading the project clinical team and the whole project team when it is included in his range of responsibilities.
Clinical research associate
The Clinical Research Associate ensures that the Monitoring activity of Clinical Studies is made in accordance with the protocol, study specific procedures, Good Clinical Practices rules (GCP), Pharmacovigilance requirements and applicable laws.
Clinical trial assistant
The Clinical Trial assistant is responsible for ensuring the management of the essential documents for clinical trials in accordance with Good Clinical Practice ICH, applicable local regulation and applicable procedures for the project.
Supply Chain Management
Our data specialists can collect, analyze and interpret the data to bridge the gap between your DCT and real-world practice. Focused on the clinical supply chain activities for pharmaceuticals, the Supply Chain Manager ensures the right product in the right place at the right time for the conduct of clinical trials.
Specific tools
Aixial Group works with validated IT tools that optimise the accuracy, frequency and quality of monitoring throughout the study.
CTMS
The CTMS used by Aixial Group allows our sponsors and each member of the project team to remotely obtain a clear, continuous and exhaustive overview of the clinical project status. In a few clicks all stakeholders can access the authorisation, initiation and recruitment statuses and the level of risk detected for each investigating centre. This tool also supports secure access to monitoring reports, ongoing actions and payment status of the centres throughout the whole study duration
E-TMF
This tool provides at any time a secure access to the whole essential documentation for the trial. It also facilitates the simple and secure management of the review and validation cycles for the study documents. We also offer the possibility of linking our e-TMF with our e-ISF solution (electronic Investigator Site File), which reduces the shipment costs to the sites, as well as optimising the tracking of acknowledgements of receipt and signatures by the investigators.
Risk Tracking Tool
To enable continuous and transparent monitoring of the study-related risks, we have developed our own Risk Tracking Tool. With this tool, interfaced with the RDC from study initiation, risks can be effectively monitored and appropriate mitigation actions can be applied at 3 levels: centre, country and protocol levels. In particular, this tool allows regular extraction of reports sent to the project managers and to the Sponsor in order to provide a clear vision of the ground reality
e-sign
Our electronic signature tool ensures excellent response rates by potential sites during the feasibility phase and proven responsiveness for administrative procedures involving investigators. It also facilitates the completion by investigators of electronic questionnaires during the project.
Our delivery methods

Technical assistance
Technical support, advice and expertise

Platform
A range of services dedicated to your activities driven by our team

Projects
Performance with commitment to results
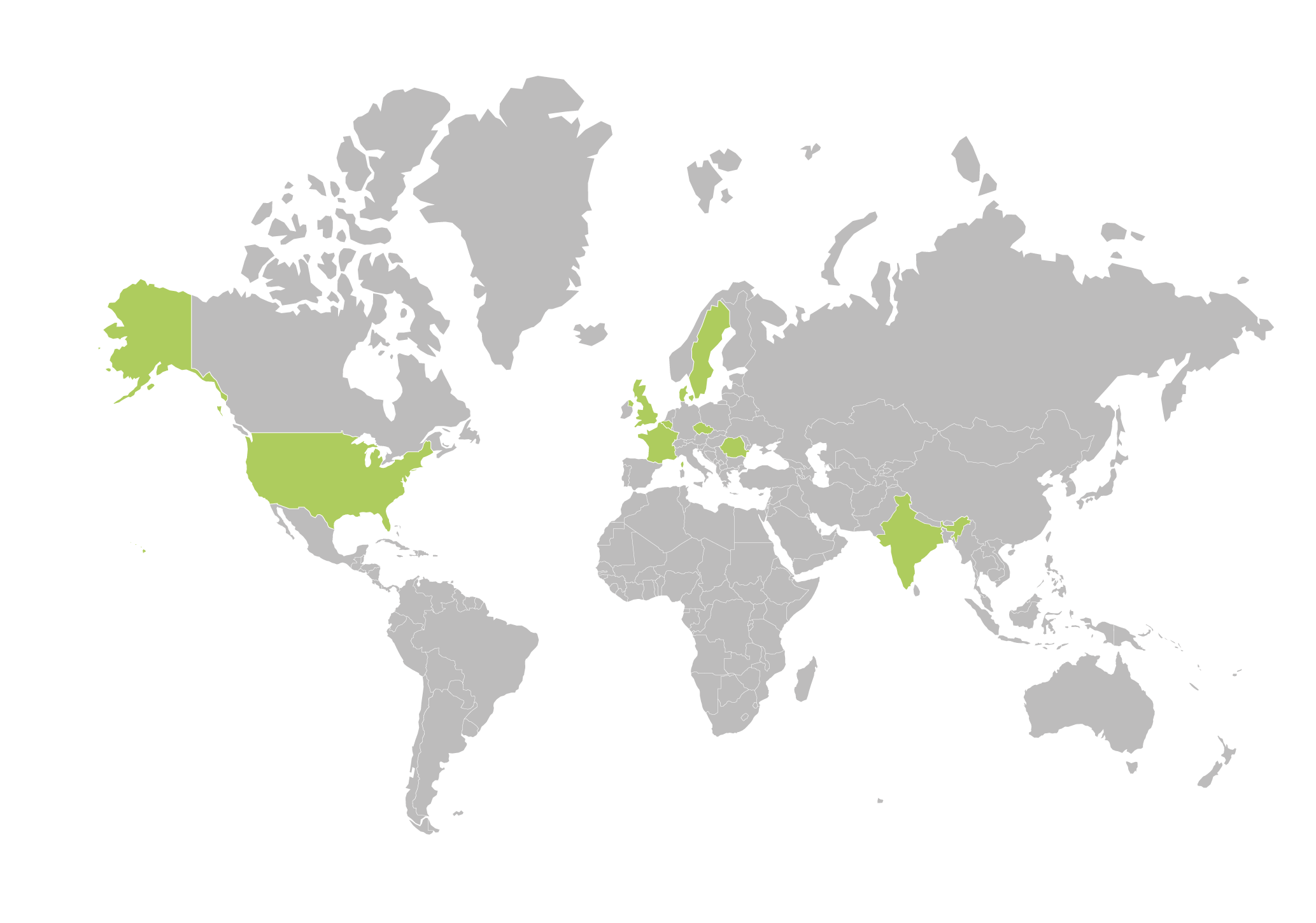
Aixial Group around the world
Aixial Group is geographically positioned to help facilitate your clinical trial wherever your research takes you. We provide services onshore, nearshore, and offshore to ensure that your solution is customized to your specific needs.
Our sectors

Pharmaceutical

Food
Biotech & Vaccines

Medical devices

Veterinary

Cosmetic
How can we support your next project?

OTHER WAYS WE SUPPORT YOU
